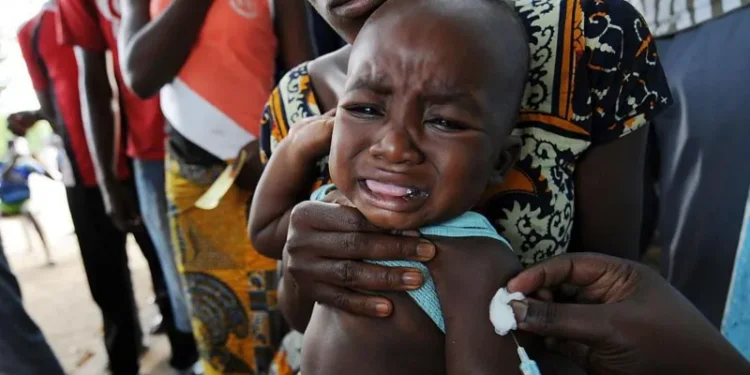
The World Health Organisation (WHO) has criticized a planned US-funded hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau, calling it “unethical”.
The trial, funded by the US Centers for Disease Control and Prevention, aimed to test the vaccine’s broader health effects by giving one set of babies the vaccine at birth and delaying it for another group until six weeks.
The WHO expressed concerns over the study’s scientific justification, ethical safeguards, and consistency with research standards.
The hepatitis B birth-dose vaccine is a proven, life-saving intervention, and withholding it from some newborns exposes them to “potentially irreversible harm”.
The Guinea-Bissau government suspended the trial after public outrage, with critics calling it unacceptable to use the country’s population as “guinea pigs”.
The WHO recommends hepatitis B vaccination at birth, and the country plans to introduce it nationwide by 2028.